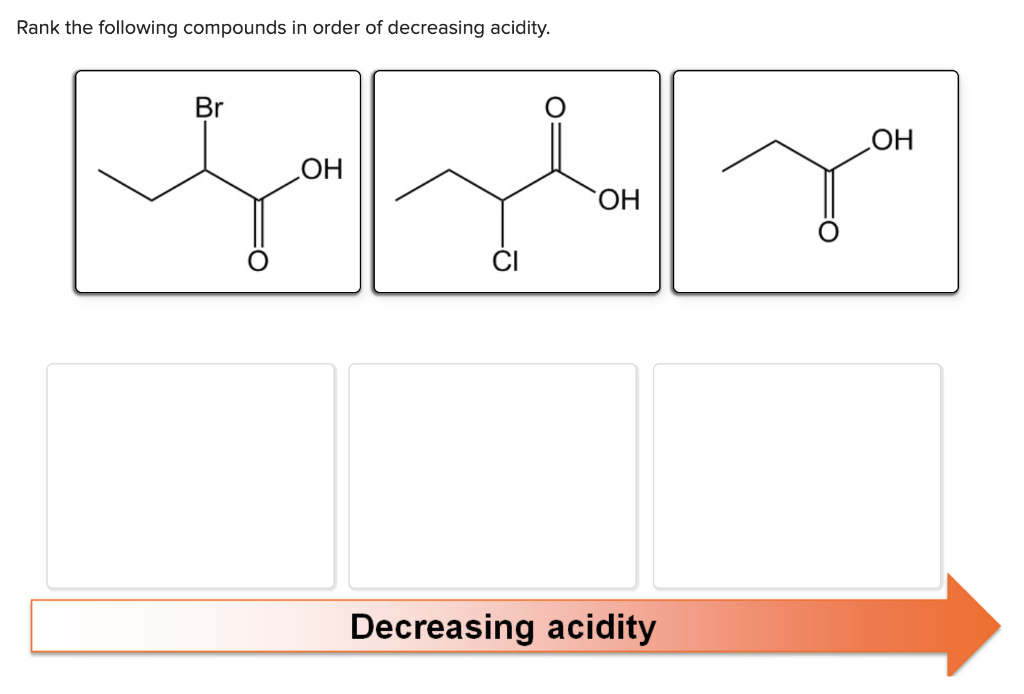
Rank the Following Compounds in Order of Decreasing Acidity.
Since presence of NO 2. CH4 NH3 HF H20 I II III IV Multiple Choice IV II L III IV T.
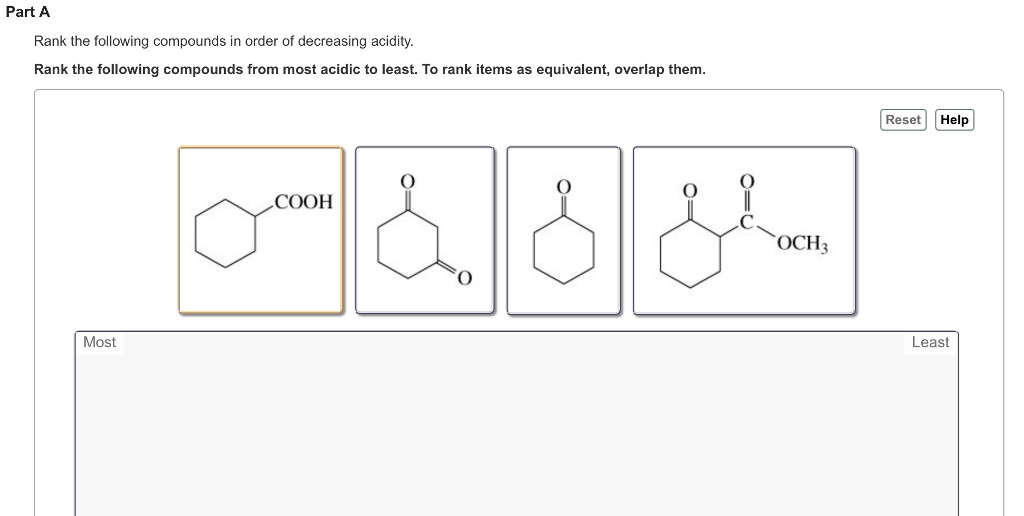
Solved Rank The Following Compounds In Order Of Decreasing Chegg Com
Among three bases X Y and Z the strongest one is Y and the weakest one is Z.

. Group in para position causes more electron-withdrawing. Rank the following conjugate. What is important here is the pka of the alpha hydrogens which are adjacent to the respective.
Rank the following compounds in order of decreasing acid strength using periodic trends. Rank these protons in order of decreasing acidity putting the most acidic first. Rank the following compounds in order of decreasing acidity putting the most acidic first.
Math Chemistry Biology Programming Arts History BusinessLanguage Spanish EnglishTipsReviewBlog Home Rank the following compounds decreasing order acidity. 10 pts O O O H N O OH ClH 2 C OH O 1 3 5 4 2 5. May 022022 - Rank the following compounds in order of decreasing acidity of the indicated hydrogen i ii iii aI II IIIbIII I IIcI III IIdIII II ICorrect answer is option B.
HSO4- pKa 199. In the given compounds it is the alpha-hydrogen which contributes to the acidity. Rank the acids from strongest to weakest.
These examples represent large classes of compounds that differ widely in acidity. To rank items as equivalent overlap them. Arrange the following compounds in order of decreasing acidity.
4 Rank the following compounds in order of decreasing acidity putting the most acidic first. Natalie Preston had the following transactions for Preston Business Services for Year 11 Provided services on account for 300002 Purchased 7500 of supplies on account3 At. Without looking them up rank the following compounds in decreasing order of acidity.
A CH3CHCH2 CH3CH2CH3 CH3CCH b CH3CH2CH2OH CH3CH2CO2H CH3CHClCO2H c 1. B IV III II I. II I IV III C.
A IV II III I. IV II I III B. Rank the following compounds in order of decreasing acidity putting the most acidic first.
CHINH CHCH mwo e acids that appeared in the preceding ans according to decreasing acidity. Rank the following compounds in order of increasing acidity putting the least acidic first. A IV II III I C III IV II I B IV III II I D III IV I II Page 4.
The decreasing order for the acid strength has been HBr HCl. Rank their conjugate acids HX HY and HZ in order of decreasing strength. Asked Aug 24 2019 in Chemistry by Danielle.
Rank the following compounds in order of decreasing acidity with 1 being the most acidic and 5 being the least acidic. III I II. Further presence of electron withdrawing group and its stronger effect stabilizes the phenoxide more.
D III IV I II. Write a short proposal. II I III IV D.
The acid strength has been defined as the dissociation of the compound for the release of hydrogen ion. C III IV II I. In acid base reactionreaction takes place in the direction where weak.
Which of the following monoprotic acids would give a solution with the lowest pH at the same molarity. Rank the following compounds in order of decreasing acid. Rank the following compounds in order of decreasing acidity putting the most acidic.
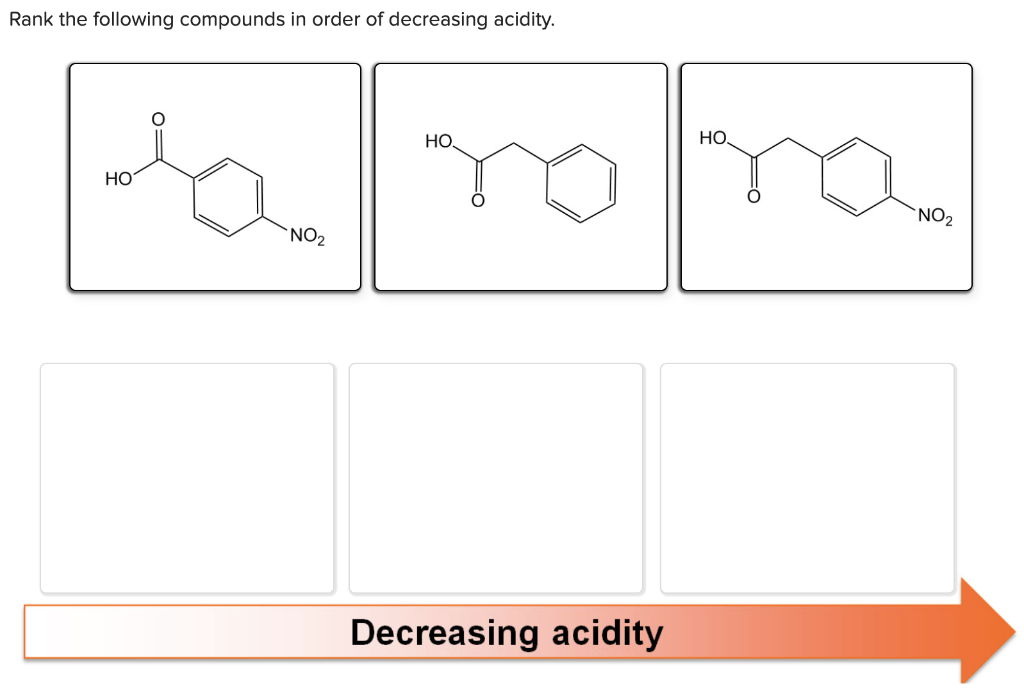
Solved Rank The Following Compounds In Order Of Decreasing Chegg Com
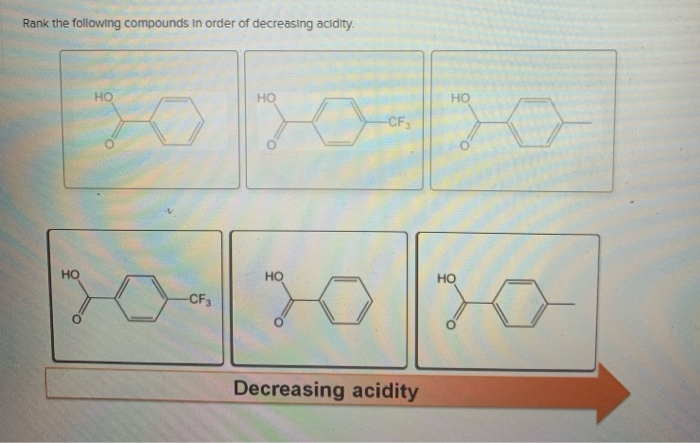
Solved Rank The Following Compounds In Order Of Decreasing Chegg Com
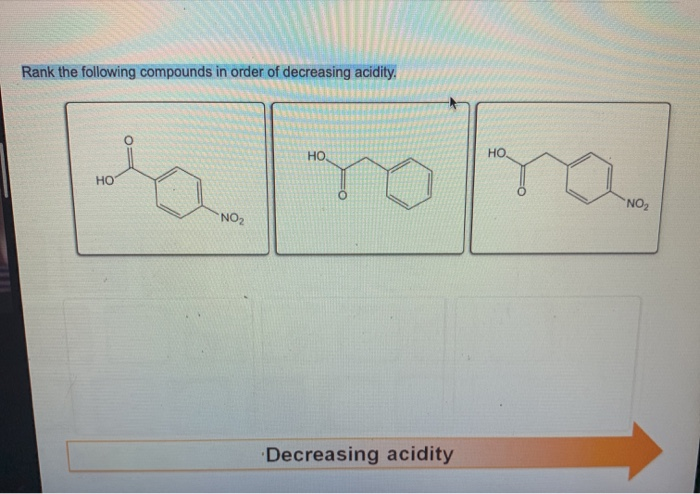
Solved Rank The Following Compounds In Order Of Decreasing Chegg Com
Solved Rank The Following Compounds In Order Of Decreasing Chegg Com
Comments
Post a Comment